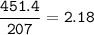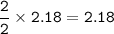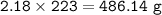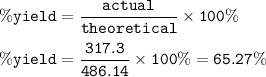Consider the reaction.
2Pb(s)+O2(g)⟶2PbO(s)
An excess of oxygen reacts with 451.4 g of...


Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Questions in other subjects:


Mathematics, 08.07.2019 05:00




History, 08.07.2019 05:00

Geography, 08.07.2019 05:00


Mathematics, 08.07.2019 05:00