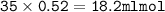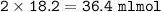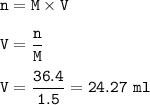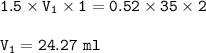Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
HELP In a neutralization reaction, aqueous HCl and aqueous Ba(OH)2 react to form aqueous BaCl2 and w...
Questions in other subjects:


Mathematics, 17.01.2021 03:30



History, 17.01.2021 03:40

Spanish, 17.01.2021 03:40


History, 17.01.2021 03:40

Mathematics, 17.01.2021 03:40

Physics, 17.01.2021 03:40