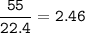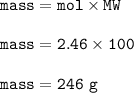Chemistry, 07.12.2020 06:00 nails4life324
When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reaction
CaCO3(s)→CaO(s)+CO2(g)
What is the mass of calcium carbonate needed to produce 55.0 L of carbon dioxide at STP?
Express your answer with the appropriate units.
mass of CaCO3 =

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reac...
Questions in other subjects:


World Languages, 08.12.2021 14:00


Mathematics, 08.12.2021 14:00


History, 08.12.2021 14:00

Geography, 08.12.2021 14:00



English, 08.12.2021 14:00