
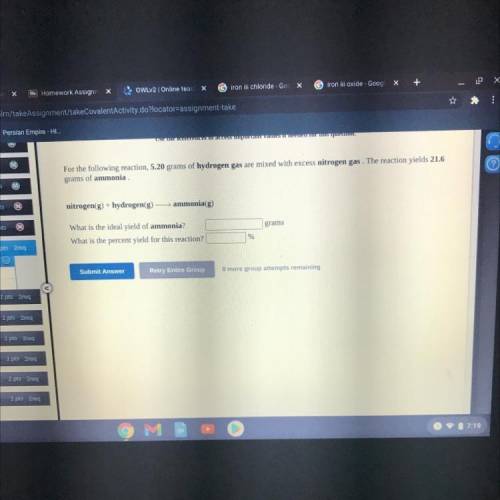
For the following reaction, 5.20 grams of hydrogen gas are mixed with excess nitrogen gas. The reaction yields 21.6
grams of ammonia
nitrogen(g) + hydrogen(g) — ammonia(g)
grams
What is the ideal yield of ammonia?
What is the percent yield for this reaction?
%
Submit Answer
Retry Entire Group
8 more group attempts remaining

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3


Chemistry, 23.06.2019 03:00, cabreradesirae4807
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
You know the right answer?
For the following reaction, 5.20 grams of hydrogen gas are mixed with excess nitrogen gas. The react...
Questions in other subjects:


History, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20


Mathematics, 30.04.2021 05:20


English, 30.04.2021 05:20

Mathematics, 30.04.2021 05:20

English, 30.04.2021 05:20



