
Chemistry, 07.12.2020 04:40 sbailey0962
According to the following reaction, how many grams of hydrogen peroxide (H2O2) are needed to form 21.9 grams of oxygen gas?
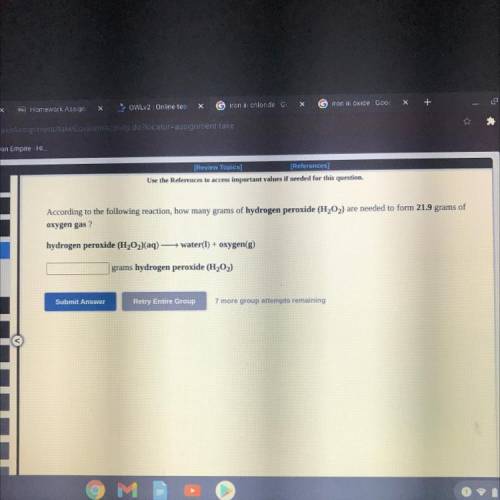

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1


Chemistry, 22.06.2019 23:00, catdog5225
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
According to the following reaction, how many grams of hydrogen peroxide (H2O2) are needed to form 2...
Questions in other subjects:

Mathematics, 21.03.2020 20:08

Mathematics, 21.03.2020 20:08


English, 21.03.2020 20:09



English, 21.03.2020 20:10

Mathematics, 21.03.2020 20:10

Social Studies, 21.03.2020 20:10



