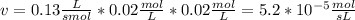Chemistry, 18.08.2019 12:00 emiliapizzillo
The rate law for a hypothetical reaction is rate = k [a][b]. if the concentrations of a and b are both 0.020 moles per liter and k = 1.3 × 10-1 m-1s-1, what is the reaction rate?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, nique0808
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2


Chemistry, 23.06.2019 04:10, angelina6836
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2
You know the right answer?
The rate law for a hypothetical reaction is rate = k [a][b]. if the concentrations of a and b are bo...
Questions in other subjects:





Mathematics, 29.11.2021 21:50


Chemistry, 29.11.2021 21:50