
Chemistry, 06.12.2020 19:00 alanflores40
The pH of a 0.15 M butylamine, C&H3NH2 solution is 12.0 at 25°C. Calculate the dissociation constant of the base.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, waterborn7152
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
The pH of a 0.15 M butylamine, C&H3NH2 solution is 12.0 at 25°C. Calculate the dissociation
con...
Questions in other subjects:






Physics, 08.12.2019 09:31

Mathematics, 08.12.2019 09:31


Mathematics, 08.12.2019 09:31

Geography, 08.12.2019 09:31

![\rm Kb=\dfrac{[L][OH^-]}{[LOH]}](/tpl/images/0954/6725/84d1f.png)
![\tt [OH^-]=\sqrt{Kb.M}](/tpl/images/0954/6725/ab388.png)
![\tt [OH^-]=10^{-pOH}\\\\(OH^-]=10^{-2}](/tpl/images/0954/6725/b2b50.png)
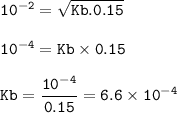
![\tt Kb=\dfrac{x^2}{0.15-x}\rightarrow x=[OH^-]\\\\Kb=\dfrac{10^{-4}}{0.15-10^{-2}}=7.14\times 10^{-4}](/tpl/images/0954/6725/69346.png)


