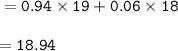Chemistry, 06.12.2020 16:50 davienwatson8
A sample of fluorine contains isotopes with different masses; F-19 and F-18. In the sample 94% of the atoms are F-19. Calculate the relative mass of the sample.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1
You know the right answer?
A sample of fluorine contains isotopes with different masses; F-19 and F-18. In the sample 94% of th...
Questions in other subjects:


Chemistry, 04.02.2020 01:02

Mathematics, 04.02.2020 01:02

Arts, 04.02.2020 01:02

Mathematics, 04.02.2020 01:02



Arts, 04.02.2020 01:03