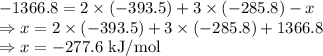Chemistry, 06.12.2020 05:40 sebastianapolo5
Ethanol undergoes combustion in oxygen to produce carbon dioxide gas and liquid water. The standard heat of combustion of ethanol, c2h5oh(l), is -1366.8 JK/mol. Given that [co2(g)] = -393.5kg/mol and [h20(l) = -285.8 KJ/ mol, what is the standard enthalpy of formation of ethanol?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:40, jeffcarpenter
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 23.06.2019 03:50, KAITLYN007
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

Chemistry, 23.06.2019 08:10, andrewrangel63
An experiment is conducted to see if cats preferred skim milk or 2% milk. a cup of skim milkwas put out for 5 kittens and then measured how much the kittens drank over the course of aday. following a cup of 2% milk was purout for the skittens and then masured how much thekittens drank over the course of a day. the same kittens were used and the milk was served atthe same temperature. it was discovered that the cats liked the 2% milk more than the skimmilk. what is the dependent variable in this experiment?
Answers: 1
You know the right answer?
Ethanol undergoes combustion in oxygen to produce carbon dioxide gas and liquid water. The standard...
Questions in other subjects:


Social Studies, 06.05.2020 09:59


English, 06.05.2020 09:59



Health, 06.05.2020 09:59



Mathematics, 06.05.2020 09:59

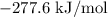
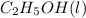 = -1366.8 kJ/mol
= -1366.8 kJ/mol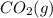 = -393.5 kJ/mol
= -393.5 kJ/mol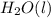 = -285.8 kJ/mol
= -285.8 kJ/mol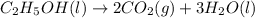
 is the standard enthalpy of formation of ethanol
is the standard enthalpy of formation of ethanol