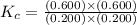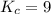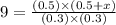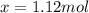Chemistry, 09.12.2019 05:31 wednesdayA
An equilibrium mixture contains 0.600 mol of each of the products (carbon dioxide and hydrogen gas) and 0.200 mol of each of the reactants (carbon monoxide and water vapor) in a 1.00-l container. this is the equation: co(g)+h2o(> < -- co2(g) + h2(g). how many moles of carbon dioxide would have to be added at constant temperature and volume to increase the amount of carbon monoxide to 0.300 mol?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3


Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
You know the right answer?
An equilibrium mixture contains 0.600 mol of each of the products (carbon dioxide and hydrogen gas)...
Questions in other subjects:



Advanced Placement (AP), 02.03.2021 17:40





Biology, 02.03.2021 17:40


Chemistry, 02.03.2021 17:40

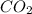 added will be 1.12 mole.
added will be 1.12 mole.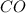 and
and 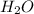 at equilibrium = 0.200 mol
at equilibrium = 0.200 mol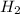 at equilibrium = 0.600 mol
at equilibrium = 0.600 mol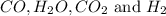 at equilibrium.
at equilibrium.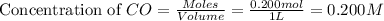
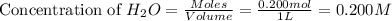
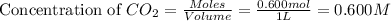
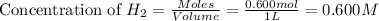
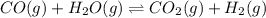
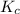 will be,
will be,![K_c=\frac{[H_2][CO_2]}{[CO][H_2O]}](/tpl/images/0409/6473/e1151.png)