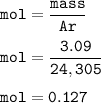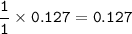Chemistry, 05.12.2020 03:40 willcoop6470
In the lab, a student collects hydrogen gas over water in a eudiometer. The hydrogen gas is produced when a piece of magnesium metal reacts with excess hydrochloric acid. Part 1: (a) Write the balanced chemical equation for this reaction. Include the states of matter. Mg(s) + 2HCl (aq) — H2 (8) +MgCl, (aq) Part 2 out of 2 (b) How many moles of hydrogen gas are collected if 3.09 g of magnesium metal is used in the reaction? Report prc Moles of hydrogen gas mol H2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, deanlmartin
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 07:40, sadcase85
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na, so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1
You know the right answer?
In the lab, a student collects hydrogen gas over water in a eudiometer. The hydrogen gas is produced...
Questions in other subjects:


Mathematics, 12.12.2020 16:40

History, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40


Social Studies, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Computers and Technology, 12.12.2020 16:40