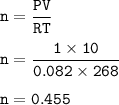Chemistry, 04.12.2020 14:00 littlemrslazy
Suppose that 10.0 L of Carbon Dioxide gas are produced by this reaction, 4C3H5N3O9 -> 12 CO2 + 10H2O + 6N2 +O2, at a temperature of -5 degrees C, and a pressure of exactly 1 atm. Calculate the mass of nitroglycerin that must have reacted in grams.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, kevinhill185
Pauling and lewis questioned the extreme definitions of bonds. they wondered if bonds might be described somewhere in between the two extremes (covalent and ionic). on the basis of experimental data, pauling confirmed that bonds could be ionic, covalent, and for those, in between, exhibit a degree of ionic character. he theorized that the major factor was how strongly the atoms in the bond attracted the electrons. pauling called this factor - the tendency of an atom to attract electrons in a bond.
Answers: 2

Chemistry, 22.06.2019 00:00, annsmith66
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 04:00, BaileyElizabethRay
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3
You know the right answer?
Suppose that 10.0 L of Carbon Dioxide gas are produced by this reaction, 4C3H5N3O9 -> 12 CO2 + 10...
Questions in other subjects:

History, 14.10.2019 00:50

Mathematics, 14.10.2019 00:50


History, 14.10.2019 00:50

Chemistry, 14.10.2019 00:50


Geography, 14.10.2019 00:50


Mathematics, 14.10.2019 00:50