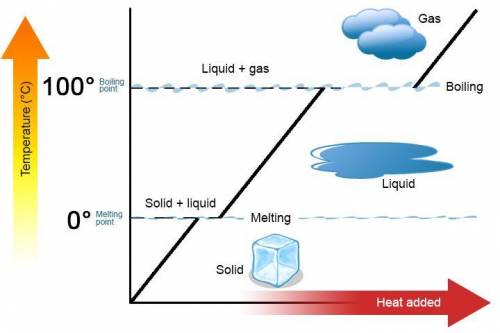
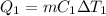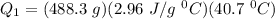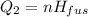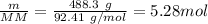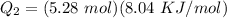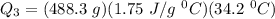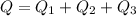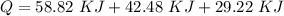A 488.3 gram sample of an unknown substance (MM = 92.41 g/mol) is heated from -23.1 °C to 51.8 °C. (heat capacity of solid = 2.96 J/g・°C; heat capacity of liquid = 1.75 J/g・°C; ∆Hfus = 8.04 kJ/mol; Tfinal = 17.6 °C)
a) How much energy (in kJ) is absorbed/released to heat the solid?
b)How much energy (in kJ) is absorbed/released to melt the solid?
c)How much energy (in kJ) is absorbed/released to heat the liquid?
d) What is the total amount of energy that must be absorbed/released for the entire process?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2


Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
A 488.3 gram sample of an unknown substance (MM = 92.41 g/mol) is heated from -23.1 °C to 51.8 °C. (...
Questions in other subjects:

English, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00

English, 10.12.2020 14:00

History, 10.12.2020 14:00


Mathematics, 10.12.2020 14:00

Spanish, 10.12.2020 14:00

Chemistry, 10.12.2020 14:00

Chemistry, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00