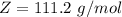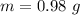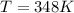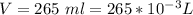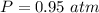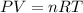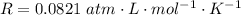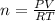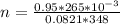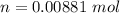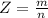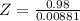Chemistry, 03.12.2020 19:50 nanagardiner08
A 0.98 gram sample of a volatile liquid was heated to 348 k. the gas occupied 265 ml of space at a pressure of 0.95 atm. what is the molecular weight of this gas?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
A 0.98 gram sample of a volatile liquid was heated to 348 k. the gas occupied 265 ml of space at a p...
Questions in other subjects: