
Chemistry, 03.12.2020 18:40 rjennis002
The following reaction shows sodium carbonate reacting with calcium hydroxide.
Na2CO3 + Ca(OH)2 → 2NaOH + CaCO3
How many grams of NaOH are produced from 20.0 grams of Na2CO3?
(Molar mass of Na = 22.989 g/mol, C = 12.01 g/mol, O = 15.999 g/mol, Ca = 40.078 g/mol, H = 1.008 g/mol) (5 points)
a. 12.2 grams
b. 15.1 grams
c. 24.4 grams
d. 30.2 grams

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, alexandraparava
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1


Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1
You know the right answer?
The following reaction shows sodium carbonate reacting with calcium hydroxide.
Na2CO3 + Ca(OH)2 → 2...
Questions in other subjects:

Mathematics, 24.04.2020 01:59




Social Studies, 24.04.2020 01:59



History, 24.04.2020 01:59

Mathematics, 24.04.2020 01:59

History, 24.04.2020 01:59

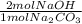 = 0.378 moles NaOH
= 0.378 moles NaOH

