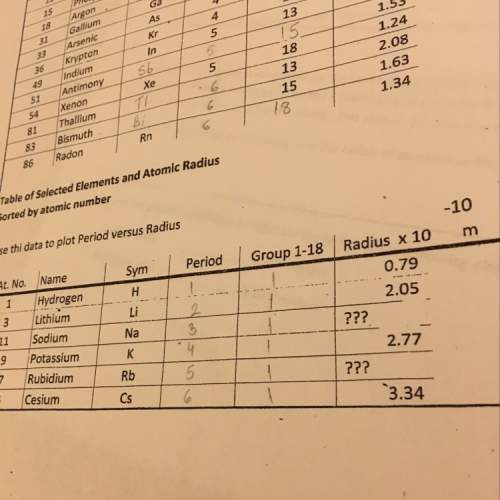
Chemistry, 03.12.2020 08:30 kolbehoneyman
Calculate the atomic mass of copper if copper-63 is 69.17% abundant and copper-65 is 30% abundant

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, badgirl2005
This is a mixture that has the same composition throughout.
Answers: 1


Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1
You know the right answer?
Calculate the atomic mass of copper if copper-63 is 69.17% abundant and copper-65 is 30% abundant...
Questions in other subjects:



History, 08.10.2020 01:01

Mathematics, 08.10.2020 01:01


Health, 08.10.2020 01:01

English, 08.10.2020 01:01

Engineering, 08.10.2020 01:01