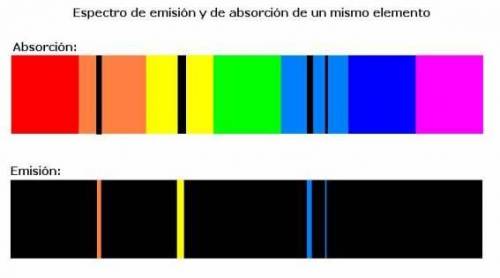
Chemistry, 03.12.2020 07:00 alexreddin3127
What does an atomic emission spectrum look like if the electrons energy levels in an atom were not quantizied
a. lines would be shifted into the ultraviole region
b. there would be fewer lines
c. there will be more lines
d. the spectrum would be constnuous

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1


Chemistry, 23.06.2019 04:00, anonymous1813
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
What does an atomic emission spectrum look like if the electrons energy levels in an atom were not q...
Questions in other subjects:

Physics, 17.10.2021 14:00


Chemistry, 17.10.2021 14:00

English, 17.10.2021 14:00



Business, 17.10.2021 14:00

Mathematics, 17.10.2021 14:00