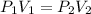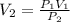Chemistry, 03.12.2020 01:00 chloebaby8
6.0L of a gas exert a pressure of 2.5 atm. When the pressure is increased to 10.0atm what is the new volume of the gas

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2


Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
6.0L of a gas exert a pressure of 2.5 atm. When the pressure is increased to 10.0atm what is the new...
Questions in other subjects:



World Languages, 03.04.2020 03:41


Mathematics, 03.04.2020 03:42





Advanced Placement (AP), 03.04.2020 03:43