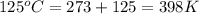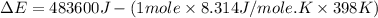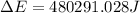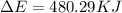Chemistry, 31.08.2019 00:00 nathaniel12
Consider the reaction: 2 h2o (> 2 h2 (g) + o2 (g). δh=483.6 kj/mol. if 2 moles of h2o (g) are converted h2(g) and o2(g) against a pressure of 1 atm at 125 degrees celcius what is δe of reaction?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1


Chemistry, 22.06.2019 23:00, aly02
Need asap question 1 minerals are organic compounds. true false question 2 what vitamin can be found in foods like oranges, grapefruits, and broccoli? a. vitamin a b. vitamin k c. vitamin c d. vitamin d question 3 what are minerals? a. chemical elements that are needed for body processes. b. organic compounds that the body needs in small amounts to function properly. c. small molecules used to build proteins. d. an organic compound that is insoluble in water and includes fats. question 4 how many types of vitamins does the human body need? a. 15 b. 11 c. 13 d. 17 question 5 vitamins are a good source of energy. true false
Answers: 1
You know the right answer?
Consider the reaction: 2 h2o (> 2 h2 (g) + o2 (g). δh=483.6 kj/mol. if 2 moles of h2o (g) are co...
Questions in other subjects:


Mathematics, 25.06.2020 03:01

Social Studies, 25.06.2020 03:01


Mathematics, 25.06.2020 03:01

Mathematics, 25.06.2020 03:01

Health, 25.06.2020 03:01

English, 25.06.2020 03:01


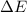 of the reaction is, 480.29 KJ.
of the reaction is, 480.29 KJ.
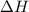 = enthalpy of the reaction = 483.6 KJ/mole = 483600 J
= enthalpy of the reaction = 483.6 KJ/mole = 483600 J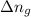 = change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole
= change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole