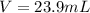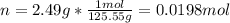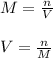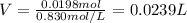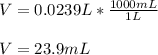Chemistry, 02.12.2020 14:00 isabelperez063
1. If 2.49 g of CuNO3 is dissolved in water to make a 0.830 M solution, what is the volume of the solution in milliliters?
2. How many moles of NaOH are present in 13.5 mL of 0.170 M NaOH?
3. Calculate the molarity of 0.650 mol of Na2S in 1.15 L of solution.
4. A student in lab needs to make a solution that is 7.00% by mass NaCl. If 145 g of NaCl is available, what mass of solution can be prepared?
5. Calculate the molarity of 24.1 g of MgS in 777 mL of solution.
6. A solution made by adding 16.3mL of methyl alcohol to enough water to give 541
mL of solution.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1


Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
1. If 2.49 g of CuNO3 is dissolved in water to make a 0.830 M solution, what is the volume of the so...
Questions in other subjects: