Chemistry, 23.12.2019 04:31 pearlkissp1bzl8
Calculate the change in internal energy of the following system: a 100.0-g bar of gold is heated from 25 ∘c to 50 ∘c during which it absorbs 322 j of heat. assume the volume of the gold bar remains constant.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2


You know the right answer?
Calculate the change in internal energy of the following system: a 100.0-g bar of gold is heated fr...
Questions in other subjects:

History, 24.04.2021 02:40







English, 24.04.2021 02:40

Chemistry, 24.04.2021 02:40

Mathematics, 24.04.2021 02:40

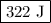
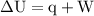 …… (1)
…… (1)
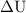 is the change in internal energy of the system.
is the change in internal energy of the system.
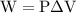 …… (2)
…… (2)
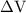 is the change in the volume of the system.
is the change in the volume of the system.