

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1


Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
Starting with 1.5052g of bacl2•2h2o and excessh2so4, how many grams of baso4 can be formed?...
Questions in other subjects:


Mathematics, 16.10.2020 19:01

Mathematics, 16.10.2020 19:01


English, 16.10.2020 19:01

History, 16.10.2020 19:01


French, 16.10.2020 19:01


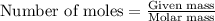 .....(1)
.....(1)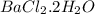 = 1.5052 g
= 1.5052 g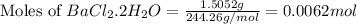
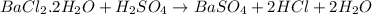
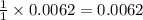 moles of barium sulfate
moles of barium sulfate


