
Chemistry, 01.12.2020 03:20 christianfielding336
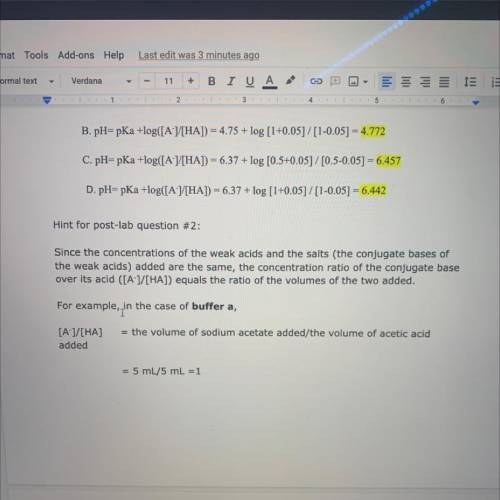
Hint for post-lab question #2:
Since the concentrations of the weak acids and the salts (the conjugate bases of
the weak acids) added are the same, the concentration ratio of the conjugate base
over its acid ([A-]/[HA]) equals the ratio of the volumes of the two added.
For example, in
in the case of buffer a,
= the volume of sodium acetate added/the volume of acetic acid
[A-]/[HA]
added
= 5 mL/5 mL =1

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 23.06.2019 01:30, kcarstensen59070
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 04:31, cassiuspricerules
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
Hint for post-lab question #2:
Since the concentrations of the weak acids and the salts (the conjug...
Questions in other subjects:












