
Chemistry, 30.11.2020 22:00 shanedietz44ovi6rb
1)26.4 % Carbon
3.3 % Hydrogen
70.3 % Oxygen
Molar Mass: 91.0 g/mol
Empirical Formula:
Molecular Formula:

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 01:40, mandilynn22
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Chemistry, 24.06.2019 01:00, emma3216
Janelle thinks that genetic engineering could be used to make a larger fruit rather than cloning the fruit. which would best support her point of view? genetic engineering does not cost as much. genetic engineering does not result in genetically identical organisms. genetically engineered foods never cause allergies in people. genetically engineered foods have not been modified.
Answers: 1
You know the right answer?
1)26.4 % Carbon
3.3 % Hydrogen
70.3 % Oxygen
Molar Mass: 91.0 g/mol
Empirical For...
70.3 % Oxygen
Molar Mass: 91.0 g/mol
Empirical For...
Questions in other subjects:

Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01


English, 12.10.2020 01:01


Mathematics, 12.10.2020 01:01

Spanish, 12.10.2020 01:01



Computers and Technology, 12.10.2020 01:01

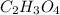 Molecular Formula: (
Molecular Formula: (


