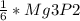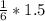Chemistry, 30.11.2020 18:20 descampbell2001
2. 1.5 moles of AgNO3 reacts with 0.5 mole of Mg3P2. Calculate the moles of excess reactant that remains at the end of the reaction. Include math to justify your answer.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 23.06.2019 01:30, AptAlbatross
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
2. 1.5 moles of AgNO3 reacts with 0.5 mole of Mg3P2. Calculate the moles of excess
reactant that re...
Questions in other subjects:

Advanced Placement (AP), 03.08.2020 14:01

History, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01


Social Studies, 03.08.2020 14:01