
Chemistry, 30.11.2020 04:30 brianamarialove15
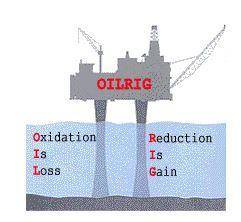
Idenify each statement below as either oxidation or reduction.
1.
Occurs when atoms gain electrons during bonding.:
2.
Occurs when the oxidation number of an element decreases in chemical reaction.:
3.
Occurs when atoms lose electrons during bonding.
4.
F + e- -> F-
5.
Occurs when the oxidation number of an element increases in chemical reaction.:
Occurs when the oxidation number of an element increases in chemical reaction.
6.
2I- -> I2 + 2e-

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1


Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Idenify each statement below as either oxidation or reduction.
1.
Occurs when atoms gain...
Occurs when atoms gain...
Questions in other subjects:

English, 05.07.2019 02:00

History, 05.07.2019 02:00

Social Studies, 05.07.2019 02:00


Mathematics, 05.07.2019 02:00

Mathematics, 05.07.2019 02:00




Mathematics, 05.07.2019 02:10



