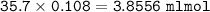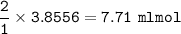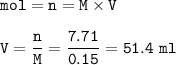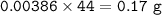Chemistry, 29.11.2020 03:20 elizabethseoane1607
Part A
What volume (in mL ) of a 0.150 M HNO3 solution will completely react with 35.7 mL of a 0.108 M Na2CO3 solution according to the following balanced chemical equation?
In the reaction in Part A, what mass (in grams) of carbon dioxide forms?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 19:00, lazavionadams81
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1


Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Part A
What volume (in mL ) of a 0.150 M HNO3 solution will completely react with 35.7 mL of a 0.10...
Questions in other subjects:

Biology, 15.01.2021 23:10

Mathematics, 15.01.2021 23:10

English, 15.01.2021 23:10

Biology, 15.01.2021 23:10


Mathematics, 15.01.2021 23:10

Mathematics, 15.01.2021 23:10

Mathematics, 15.01.2021 23:10

Biology, 15.01.2021 23:10

English, 15.01.2021 23:10