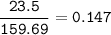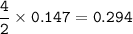Chemistry, 29.11.2020 02:40 ariloveshorses
(Need Help ASAP) 23.5 grams of iron(III) oxide (Fe2O3) are reacted with excess carbon. What is the theoretical yield of iron metal? 2 Fe2O3 + 3 C --> 4 Fe + 3 CO2

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3
You know the right answer?
(Need Help ASAP) 23.5 grams of iron(III) oxide (Fe2O3) are reacted with excess carbon. What is the t...
Questions in other subjects:

Geography, 20.02.2021 14:10

Spanish, 20.02.2021 14:10



Biology, 20.02.2021 14:10


Biology, 20.02.2021 14:10

English, 20.02.2021 14:10


Social Studies, 20.02.2021 14:10