
2. HI(g) → H2(g) + I2(g)
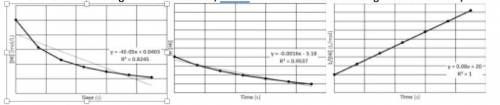
A 0.050 M sample of hydrogen iodide is decomposed into hydrogen and iodine, according to the equation above. The [HI], ln[HI], and 1/[HI] vs. time graphs are plotted. (The equations are given for the best-fit straight line for the data, and R2 value indicates the strength of correlation.)
a. Write the rate law for this reaction. Justify your response using the graphs provided.
b. Find the value of the rate constant, k. Include units.
c. At what time will [HI] be 0.001 M?
image of graphs are attached

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, esnyderquintero
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2


You know the right answer?
2. HI(g) → H2(g) + I2(g)
A 0.050 M sample of hydrogen iodide is decomposed into hydrogen and iodine...
Questions in other subjects:

Computers and Technology, 19.03.2021 18:30


Mathematics, 19.03.2021 18:30


Mathematics, 19.03.2021 18:40





Mathematics, 19.03.2021 18:40



