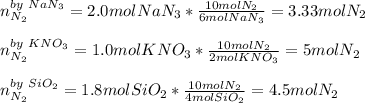Chemistry, 28.11.2020 03:20 isabellemaine
When an airbag expands in a vehicle, sodium azide reacts with potassium nitrate and silicon dioxide, releasing nitrogen gas and sodium potassium silicate (fine glass powder) 6 NaN3+ 2 KNO3 + 4 SiO210 N2+ 2 NaKSiO3+ 2 Na2SiO3 + 02 To conduct a similar reaction, 2.0 mol of NaN3, 1.0 mol of KNO3 and 1.8 mol of SiO2 are added. Which one is the limiting reactant? a) N2 b) O2 c) KNO3 d) SiO2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, baileysosmart
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3



Chemistry, 23.06.2019 02:00, hannabeth91
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
When an airbag expands in a vehicle, sodium azide reacts with potassium nitrate and silicon dioxide,...
Questions in other subjects:


Biology, 28.12.2019 11:31



Mathematics, 28.12.2019 11:31

Mathematics, 28.12.2019 11:31

Social Studies, 28.12.2019 11:31

Health, 28.12.2019 11:31