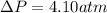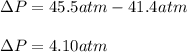Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2



Chemistry, 23.06.2019 03:30, antoinetteee03
Name atleast 3 type of energy associated with the microwave
Answers: 1
You know the right answer?
15.0 moles of gas are in a 8.00 L tank at 22.3 ∘C∘C . Calculate the difference in pressure between m...
Questions in other subjects:




Mathematics, 05.05.2020 19:21

Mathematics, 05.05.2020 19:21

Mathematics, 05.05.2020 19:21

Business, 05.05.2020 19:21


Mathematics, 05.05.2020 19:21