
Chemistry, 27.11.2020 16:20 s0cial0bessi0n
A 6g sample of carbon allowed to burn in 20g of oxygen gas produce carbon dioxide. After the reaction, the mass of unreacted oxygen is 4 g. What mass of carbon dioxide was produced? show with steps

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3


Chemistry, 23.06.2019 07:30, lucas2020197
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
You know the right answer?
A 6g sample of carbon allowed to burn in 20g of oxygen gas produce carbon dioxide. After the reactio...
Questions in other subjects:

Social Studies, 17.09.2019 18:00



Health, 17.09.2019 18:00

Mathematics, 17.09.2019 18:00


Mathematics, 17.09.2019 18:00

Mathematics, 17.09.2019 18:00


 .
. :
:  .
. :
:  .
. and
and  :
: .
. .
. of
of 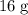 of
of 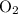 :
: .
. .
.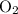 and
and  . For each mole of
. For each mole of  will be produced.
will be produced.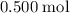 , of
, of  of
of 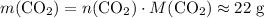 .
.

