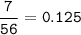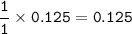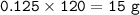Chemistry, 27.11.2020 14:00 whitakers87
Sulfur dioxide is an atmospheric pollutant.
Sulfur dioxide pollution is reduced by reacting calcium oxide with sulfur dioxide to
produce calcium sulfite.
CaO + SO2 → CaSO3
7.00 g of calcium oxide reacts with an excess of sulfur dioxide.
Relative atomic masses (Ar): O = 16 S = 32 Ca = 40 Calculate the mass of calcium sulfite produced


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, bernicewhite156
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2


Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2
You know the right answer?
Sulfur dioxide is an atmospheric pollutant.
Sulfur dioxide pollution is reduced by reacting calcium...
Questions in other subjects:

Mathematics, 25.09.2021 01:00

Biology, 25.09.2021 01:00

Mathematics, 25.09.2021 01:00






Mathematics, 25.09.2021 01:00

English, 25.09.2021 01:00