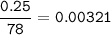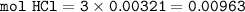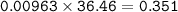Chemistry, 27.11.2020 02:00 carrieaj08
2. Al(OH),(s) + 3 HCl(aq) à 3 H2O(l) + AlCl3(aq). This reaction shows how aluminum hydroxide
in antacid tablets neutralizes hydrochloric acid in the stomach. A tablet containing 0.25 g of
aluminum hydroxide is ingested by a patient with 0.88 g of hydrochloric acid in their stomach. Is
this tablet sufficient to neutralize the acid in the patient's stomach? Explain using stoichiometric
calculations. [4 marks]
I

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, hannahmyung1113
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 23.06.2019 01:30, bgarrison364
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1

Chemistry, 23.06.2019 10:00, lexusdixon3
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1
You know the right answer?
2. Al(OH),(s) + 3 HCl(aq) à 3 H2O(l) + AlCl3(aq). This reaction shows how aluminum hydroxide
in ant...
Questions in other subjects:

Mathematics, 05.05.2020 06:51



English, 05.05.2020 06:51


Mathematics, 05.05.2020 06:51


Business, 05.05.2020 06:51