
Chemistry, 26.11.2020 21:20 Anshuman2002
Ammonia is formed from the reaction of nitrogen and hydrogen according to the equation:
N2(g) + 3H2(g) 2NH3(g)
What is the maximum number of moles of ammonia that can be formed from the reaction of 27 moles of hydrogen?
A
41
B
27
18
D
9

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
Ammonia is formed from the reaction of nitrogen and hydrogen according to the equation:
N2(g) + 3H2...
Questions in other subjects:

History, 09.11.2020 02:30



Mathematics, 09.11.2020 02:30



Arts, 09.11.2020 02:30

Mathematics, 09.11.2020 02:30

Mathematics, 09.11.2020 02:30

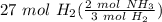 = 18 mol NH₃
= 18 mol NH₃

