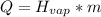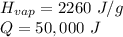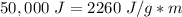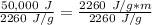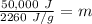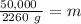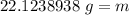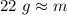Chemistry, 26.11.2020 08:20 dreamadoreaa
While you were "sweating" your chemistry test, water vapor evaporates from your body, absorbing 50,000 J of energy. (assume no
temperature change). What mass of water evaporates?
A:O 150 g
B:O 22 g
C:11,962 g
D:O 105,000 g

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, mimithurmond03
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 23.06.2019 03:00, KindaSmartPersonn
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
While you were "sweating" your chemistry test, water vapor evaporates from your body, absorbing 50,0...
Questions in other subjects:

Mathematics, 24.09.2019 18:30

Mathematics, 24.09.2019 18:30


English, 24.09.2019 18:30


English, 24.09.2019 18:30


Mathematics, 24.09.2019 18:30