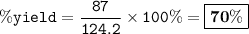Chemistry, 26.11.2020 01:00 pinkpearl20
Use the equation below to solve the problem that follows.
2H2 (g) + O2 (g) → 2H2O (g)
When David reacts 13.8 grams of hydrogen gas with excess oxygen, 87.0 grams of water are formed. Calculate his percent yield of water.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, qwerty8364
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1


Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Use the equation below to solve the problem that follows.
2H2 (g) + O2 (g) → 2H2O (g)
W...
W...
Questions in other subjects: