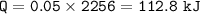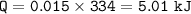PLEASE HELP WITH THESE TWO QUESTIONS
2.) 50.0 grams of water at 100 degrees Celsius evaporated to water vapor 2 points
at 100 degrees Celsius. Calculate the amount of heat required for this
conversion.
2. A student measured 15.0 grams of ice in a beaker. The beaker was then
placed on a hot plate where it was heated uniformly for a certain amount
of time. During the melting process of the ice, the student noted that the
temperature was at 0 degree Celsius. When all the ice converted to water,
the final temperature was also at 0 degree Celsius. How much heat was
used to melt the ice?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, minstcordell4115
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3


Chemistry, 21.06.2019 22:00, applereams
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1
You know the right answer?
PLEASE HELP WITH THESE TWO QUESTIONS
2.) 50.0 grams of water at 100 degrees Celsius evaporated to w...
Questions in other subjects:


Mathematics, 21.06.2019 20:30




Physics, 21.06.2019 20:30

History, 21.06.2019 20:30


Mathematics, 21.06.2019 20:30

Chemistry, 21.06.2019 20:30