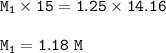Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:00, jaidencoolman2866
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1


Chemistry, 23.06.2019 09:00, aaronroberson4940
Weight is a measure of: inertia force matter mass
Answers: 1
You know the right answer?
You complete titration of 15.0ml of unknown acid with 14.16ml of 1.25 M NaOH. What’s the molar it’s...
Questions in other subjects:

Mathematics, 20.01.2021 21:00




Mathematics, 20.01.2021 21:00



Mathematics, 20.01.2021 21:00

Physics, 20.01.2021 21:00

History, 20.01.2021 21:00