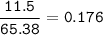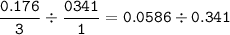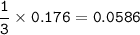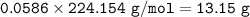Chemistry, 24.11.2020 19:10 natalia9573
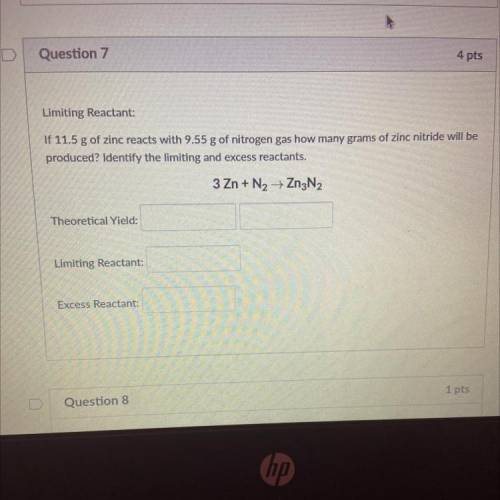
Limiting Reactant:
If 11.5 g of zinc reacts with 9.55 g of nitrogen gas how many grams of zinc nitride will be
produced? Identify the limiting and excess reactants.
3 Zn + N2 + Zn3N2
Theoretical Yield:
Limiting Reactant:
Excess Reactant:

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1
You know the right answer?
Limiting Reactant:
If 11.5 g of zinc reacts with 9.55 g of nitrogen gas how many grams of zinc nitr...
Questions in other subjects:

Health, 22.11.2021 22:30

Mathematics, 22.11.2021 22:30





Mathematics, 22.11.2021 22:30

History, 22.11.2021 22:30