
Chemistry, 24.11.2020 14:00 nagwaelbadawi
Calculate the mass in grams of carbon dioxide produced from 11.2 g of octane (C8H18) in the reaction above.
18 + 25 O2 --> 16 CO2 + 18 H2O
89.6 g
17.3 g
34.5 g
46.2 g

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, CaleWort92
Which of the following statements concerning the influence of culture on ethnic identity formation is accurate? a. one will reject ethnic identity if cultural stereotypes are encountered. b. if one’s ethnic city is different from the dominant cultural group, then one’s ethnic identity you will become weekend. c. if an the ethnic group is excepted by dominant culture, then ethnic identity formation can be a difficult process. d. similarity to the dominant culture can determine how easy it is for one to except ethnic differences.
Answers: 2

Chemistry, 22.06.2019 14:30, jessiereyes2924
What is the relationship between wind and ocean waves? question 17 options: wind moving at higher speeds will transfer more energy to the water, resulting in stronger waves. wind moving at higher speeds will transfer energy over a larger part of the ocean water, resulting in waves with a shorter wavelength. winds moving at higher speeds with cause water to move forward at faster rates, causing larger ocean waves. winds moving at higher speeds will affect deeper water, resulting in waves that move at a faster rate. how do temperature and salinity affect deepwater currents? question 15 options: as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
Calculate the mass in grams of carbon dioxide produced from 11.2 g of octane (C8H18) in the reaction...
Questions in other subjects:


Biology, 11.06.2020 21:57

English, 11.06.2020 21:57

Spanish, 11.06.2020 21:57

Mathematics, 11.06.2020 21:57



Mathematics, 11.06.2020 21:57



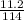 = 0.098mole
= 0.098mole  = 0.79mole of CO₂
= 0.79mole of CO₂


