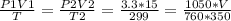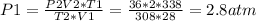2) A gas takes up a volume of 15 liters, has a pressure of 3.3 atm, and a temperature of
299 K. If I raise the temperature to 350 K and lower the pressure to 1050 mmHg, what is the
new volume of the gas?
3) A gas that has a volume of 28 liters, a temperature of 65 °C, and an unknown pressure
has its volume increased to 36 liters and its temperature decreased to 35 °C. If I measure the
pressure after the change to be 2.0 atm, what was the original pressure of the gas?
work too pls

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, netflixacc0107
Achemist requires 6.00 liters of 0.320 m h2so4 solution. how many grams of h2so4 should the chemist dissolve in water? 121 grams 159 grams 176 grams 188 grams
Answers: 2

Chemistry, 21.06.2019 18:00, 767sebmont
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2
You know the right answer?
2) A gas takes up a volume of 15 liters, has a pressure of 3.3 atm, and a temperature of
299 K. If...
Questions in other subjects:

English, 12.01.2021 04:00

Mathematics, 12.01.2021 04:00


Biology, 12.01.2021 04:00


English, 12.01.2021 04:00


Mathematics, 12.01.2021 04:00


Mathematics, 12.01.2021 04:00