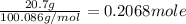Calcium carbonate decomposes into calcium oxide and carbon dioxide as shown below. If 20.7 grams of CaCO3 decompose, what is the theoretical yield in grams of CaO?
If 6.81 grams of CaO are actually recovered, what is the percent yield?
CaCO3 --> CaO + CO2
**Your answer should be written as XX. X

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, FloweyFlower
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3


Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Calcium carbonate decomposes into calcium oxide and carbon dioxide as shown below. If 20.7 grams of...
Questions in other subjects:


Mathematics, 21.05.2020 05:59



Physics, 21.05.2020 05:59

Mathematics, 21.05.2020 05:59


Mathematics, 21.05.2020 05:59


Mathematics, 21.05.2020 05:59