

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, kitykay2776
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1


Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 07:20, rscvsdfsrysas1857
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1
You know the right answer?
What is the value of delta Hrxn for this equation:
NH3(g)+2O2(g)⟶HNO3(g)+H2O(g)
Based...
Based...
Questions in other subjects:


Mathematics, 30.01.2020 10:46



English, 30.01.2020 10:46

Mathematics, 30.01.2020 10:46

Mathematics, 30.01.2020 10:46



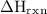 of the reaction can be 179 kJ/mol.
of the reaction can be 179 kJ/mol. 390
390 

