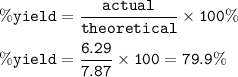At high temperatures, sulfur combines with iron to form brown-black iron(II) sulfide:
Fe(s) + SD-Fes(s)
In an experiment, 5.00 g of iron reacts with excess sulfur to produce 6.29 g of iron(II) sulfide. Calculate the percent yield if the maximum
amount of iron(II) sulfide that can be produced is 7.87 g.
79.796
79.9%
100%
63.5%
125%

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 00:40, draveon353
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 14:30, KennyOaks6230
Which of the following units is not an official si unit
Answers: 1
You know the right answer?
At high temperatures, sulfur combines with iron to form brown-black iron(II) sulfide:
Fe(s) + SD-Fe...
Questions in other subjects:

English, 31.01.2020 10:52

Computers and Technology, 31.01.2020 10:52

Mathematics, 31.01.2020 10:52

English, 31.01.2020 10:52

Biology, 31.01.2020 10:52



Social Studies, 31.01.2020 10:52