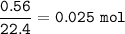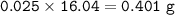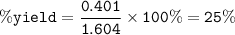Chemistry, 22.11.2020 20:50 madisongibson62
If 8.2 g of sodium ethanoate produced 560 cm3 of methane (at s. t.p.). which one of the following is the percentage yield of the reaction;
A 2.5
B 4.0
C 12.0
D 25.0

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

You know the right answer?
If 8.2 g of sodium ethanoate produced 560 cm3 of methane (at s. t.p.). which one of the following is...
Questions in other subjects:

Computers and Technology, 14.07.2019 13:00


Biology, 14.07.2019 13:00

Business, 14.07.2019 13:00

Biology, 14.07.2019 13:00

History, 14.07.2019 13:00



History, 14.07.2019 13:00

Mathematics, 14.07.2019 13:00

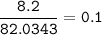
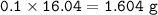 ⇒ theoretical
⇒ theoretical