

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2


Chemistry, 22.06.2019 17:10, gungamer720
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 21:00, agarcia24101993
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
How much tin II fluoride will be made when reacting 45.0 grams of tin with an excess of hydrofluoric...
Questions in other subjects:


Chemistry, 07.07.2019 01:00


English, 07.07.2019 01:00



Mathematics, 07.07.2019 01:00

Mathematics, 07.07.2019 01:00

Geography, 07.07.2019 01:00

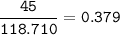
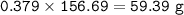 ⇒theoretical
⇒theoretical


