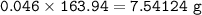Chemistry, 21.11.2020 01:50 alexismoore15567
Consider the reaction of lead(II) nitrate reacting with sodium phosphate to create lead(II) phosphate and sodium nitrate. If 37.1 g of lead(II) phosphate was created in the reaction, how much (mass) sodium phosphate was originally needed to produce this amount of product

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, RedDemon59
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
Consider the reaction of lead(II) nitrate reacting with sodium phosphate to create lead(II) phosphat...
Questions in other subjects:

Mathematics, 21.01.2021 17:10

Business, 21.01.2021 17:10

Biology, 21.01.2021 17:10

Mathematics, 21.01.2021 17:10



English, 21.01.2021 17:10

Chemistry, 21.01.2021 17:10