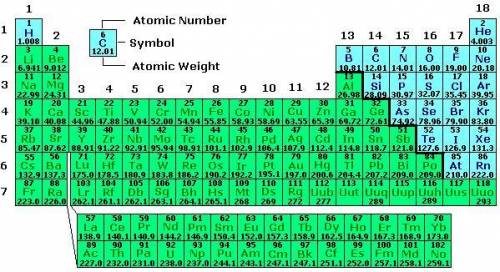
Along each row of the periodic table,
A.
atomic mass increases from left to right.
B....

Chemistry, 20.11.2020 21:00 kristieroth1
Along each row of the periodic table,
A.
atomic mass increases from left to right.
B.
atomic mass is the same for all elements in the row.
C.
atomic mass changes in no set pattern.
D.
atomic mass decreases from left to right.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2


Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Questions in other subjects:


Mathematics, 12.02.2021 22:30


Chemistry, 12.02.2021 22:30


Mathematics, 12.02.2021 22:30

English, 12.02.2021 22:30


History, 12.02.2021 22:30

Mathematics, 12.02.2021 22:30



