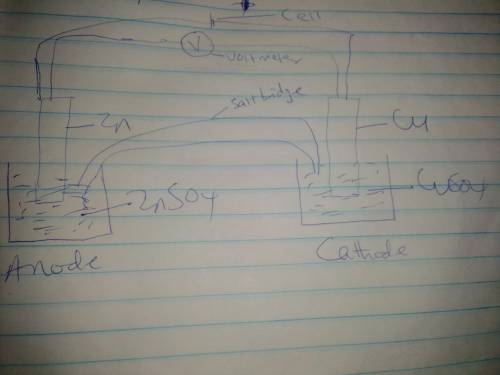
Chemistry, 20.11.2020 17:00 queenpaige2015
Use the given half reactions to "construct" an electrolytic cell. Zn^2+ + 2 e^>Zn E°cell = -0.76 V Cu^2+ + 2 e^> Cu E°cell = 0.34 V 1. Predict the standard potential of the cell at 298 K. 2. What is the minimum voltage that should be applied to the standard electrolytic cell found in question to cause zn2+ to be reduced to Zn?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:20, ineedhelp773
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases. as altitude increases, air density increases. air pressure and density are lowest at sea level. denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1

Chemistry, 23.06.2019 02:00, Hellopeople233
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
Use the given half reactions to "construct" an electrolytic cell. Zn^2+ + 2 e^>Zn E°cell = -0.76...
Questions in other subjects:


History, 25.01.2020 01:31


English, 25.01.2020 01:31